We study states of matter under chemistry. Chemistry is a branch of science that deals with structure, properties, composition, and states of matter. It deals with everything which is related to matter.
Now, let’s learn about matter, its properties, and its states.
What is the matter?
We see many things with different shapes, sizes, and textures as we look at our surroundings. Everything in this Universe comprises a material called “matter”. For example, the food we eat, chairs, the air we breathe, drops of water, soil, everything is matter.
Look around yourself and find the number of things which are made up of matter.
Physical properties of matter:
Now, let’s discuss the physical properties of matter, which are common in everything made up of matter. There are four main physical properties of matter. These are:
- Matter is made up of tiny particles. The size of the particles is so small that we cannot see them with naked eyes.
- Particles of matter have space between them.
- Particles of matter are continuously moving.
- Particles of matter attract each other.
States of matter:
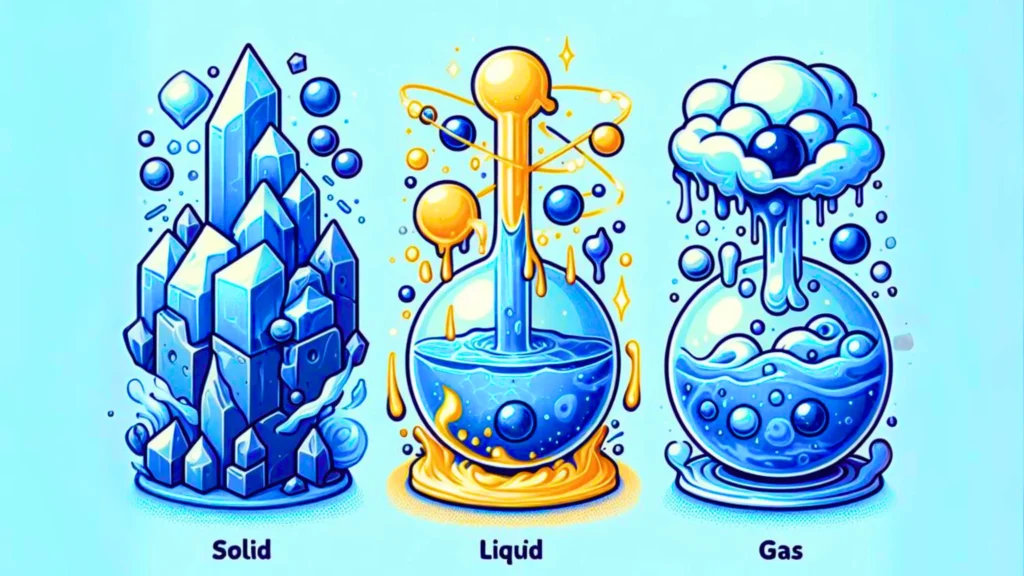
There are four states of matter. These are – solid, liquid, gas, and plasma. These are the ones that occur naturally in the Universe.
But here, we will discuss only three states of matter- solid, liquid, and gas.
The Solid State:
To understand the solid state, let’s look at the following substances: pen, book, and wooden stick. These all are examples of solid states. We can observe that all these have a definite shape, distinct boundaries, and fixed volumes.
In other words, they have negligible compressibility.
They tend to maintain their shape when subjected to outside force. Solids may break under force, but it is difficult to change their shape. So, solids are rigid.
The liquid state:
To understand the liquid state, let’s look at the following things – water, milk, juice, and cooking oil. We can observe that none of these liquids have a fixed shape but a fixed volume.
Liquids can take up the shape of the container in which they are kept. They can flow and change shape. That’s why they are not rigid, but we can call them fluid.
The Gaseous state:
Observe an inflated balloon to understand the third state of matter, which is a gaseous state. What will you see? It is filled with the third state of matter, the gaseous state.
You will also observe that gases are highly compressible as compared to solids and liquids.
The liquefied petroleum gas (LPG) cylinder that we get in our homes for cooking or the oxygen supplied to hospitals in cylinders is also compressed gas. Compressed natural gas (CNG) is used as fuel in vehicles. Due to its high compressibility, large volumes of gas can be compressed.
I hope you now know the differences between the three states of matter. We study all these three states of matter in a branch of science named chemistry. If you have any questions, please let us know in the comments!
Also Read :
5 Properties of Acids and Bases |Can Acids and Bases be stored together?
What is matter?
Everything in this Universe comprises a material called “matter”.
What are the three states of matter?
These are the three states of matter: The solid state, the liquid state, and the gaseous state.
Which state of matter has more intermolecular forces?
The solid state.
Which state of matter has a fixed shape and fixed volume?
The solid state.
Which state of matter has a fixed volume but not a fixed shape?
The liquid state.
Which state of matter neither has a fixed shape nor fixed volume?
The gaseous state.